Progesterone is a steroid hormone produced primarily by the corpus luteum that prepares the uterine lining for implantation and counter‑acts estrogen‑driven proliferation. When the body fails to maintain adequate levels - a condition known as progesterone deficiency - the delicate hormonal balance that keeps uterine tissue in check can unravel, paving the way for growths called fibroids.
Key Points
- Low progesterone removes a natural brake on estrogen, encouraging fibroid expansion.
- Fibroids are most common in women aged 35‑50 and are linked to hormonal, genetic, and lifestyle factors.
- Diagnosis relies on imaging (ultrasound, MRI) and hormonal profiling.
- Treatment options range from hormonal modulators to minimally invasive procedures.
- Managing weight, stress, and diet can support hormone balance and reduce fibroid risk.
Hormonal Landscape: Estrogen vs. Progesterone
To grasp why a progesterone shortfall matters, consider the menstrual cycle’s two main hormones. Estrogen is a polycyclic aromatic hormone secreted by the ovaries that stimulates the growth of the endometrium and drives cell proliferation in the myometrium. In a healthy cycle, rising estrogen is later tempered by progesterone, which promotes differentiation rather than growth. When progesterone lags, estrogen runs unchecked, creating an environment where smooth‑muscle cells in the uterus multiply unchecked, forming leiomyomas (fibroids).
Research from the International Society of Gynecological Endocrinology (2023) showed that women with consistently low luteal‑phase progesterone had a 2.1‑fold higher odds of developing fibroids compared with those maintaining a normal progesterone profile.
How Progesterone Deficiency Drives Fibroid Formation
The link hinges on three inter‑related mechanisms:
- Loss of anti‑proliferative signaling: Progesterone binds to the Progesterone receptor (PR), a nuclear transcription factor that activates genes slowing cell division. Fewer receptors or less hormone means the brake fails.
- Enhanced estrogen activity: Without progesterone’s moderating effect, estrogen up‑regulates growth factors like TGF‑β and IGF‑1, which stimulate extracellular matrix (ECM) deposition - a hallmark of fibroids.
- Altered aromatase expression: Low progesterone can increase ovarian and intratumoral aromatase, the enzyme that converts androstenedione to estrogen, creating a local estrogen surge that fuels fibroid growth.
In practice, a woman experiencing chronic luteal‑phase deficiencies may notice her menstrual cramps worsening, spotting becoming more frequent, and a gradual increase in pelvic pressure - all signs that fibroids might be taking hold.
Clinical Manifestations of Fibroids Linked to Low Progesterone
Uterine fibroids present with a spectrum of symptoms, many of which intensify when progesterone is low:
- Heavy menstrual bleeding (menorrhagia): Without progesterone‑mediated vascular stabilization, the endometrium can become overly fragile.
- Pelvic pain and pressure: Fibroids expand the uterus, compressing adjacent organs.
- Infertility or recurrent miscarriage: A hormonally hostile uterine environment hampers implantation.
- Urinary frequency: Large fibroids press against the bladder.
Doctors often correlate symptom severity with the size and number of fibroids, but hormonal assays revealing low progesterone can also predict rapid growth.

Diagnosing the Hormone‑Fibroid Connection
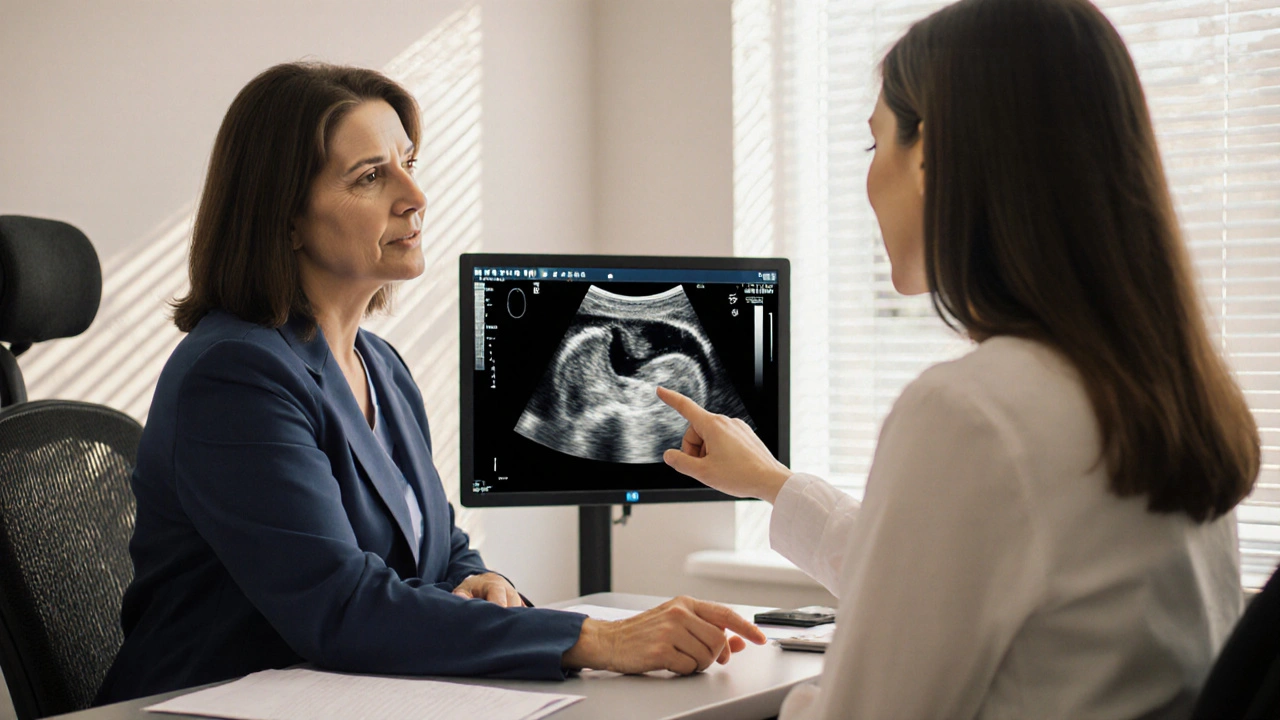
Modern gynecology combines imaging with biochemical testing. The first line is a transvaginal ultrasound exam, which visualises fibroid size, location, and vascularity. When ultrasound findings are inconclusive, a pelvic MRI scan offers higher resolution.
Simultaneously, a hormonal panel measuring luteal‑phase progesterone, estradiol, LH, and FSH helps pinpoint imbalance. Studies show that a progesterone level below 5ng/mL in the mid‑luteal window correlates with a 30% increase in fibroid growth rate over six months.
Treatment Landscape: From Hormones to Procedures
Therapeutic goals aim to restore hormonal balance, shrink fibroids, or remove them safely. Below is a comparison of the most common approaches.
| Treatment | Mechanism | Effect on Progesterone | Typical Outcome | Recovery Time |
|---|---|---|---|---|
| GnRH agonist | Suppresses pituitary GnRH → ↓ LH/FSH → temporary menopause | Drastically lowers both estrogen and progesterone | Fibroid size ↓ 30‑50% in 3‑6months | 2‑4weeks (hot flashes, bone loss risk) |
| SPRM (e.g., ulipristal) | Selectively blocks PR while allowing some progesterone action | Modulates receptor activity without dropping hormone levels | Fibroid shrinkage 20‑35%; bleeding control | 1‑2weeks; liver monitoring needed | r>
| Uterine artery embolization | Blocks blood flow to fibroids → ischemic shrinkage | Hormone levels unchanged | Average volume reduction 50‑60% over 12months | 5‑7days (post‑embolization syndrome) |
| Myomectomy | Surgical excision of fibroids | Hormones remain stable | Immediate symptom relief; fertility preservation | 2‑4weeks (laparoscopic) to 6‑8weeks (open) |
When the root problem is a progesterone shortfall, many clinicians start with a progesterone supplement - either oral micronized progesterone or a vaginal gel - to restore the natural brake. Studies in 2022 demonstrated a 15% reduction in fibroid growth rate after three months of supplementation in women with documented luteal deficiency.
Lifestyle Tweaks that Support Progesterone Production
Beyond medical therapy, everyday habits can help keep progesterone in the sweet spot:
- Maintain a healthy weight: Adipose tissue converts progesterone to estrogen via aromatase; excess fat tips the balance.
- Manage stress: Chronic cortisol can suppress luteal phase progesterone.
- Consume zinc‑rich foods (oysters, pumpkin seeds) - zinc is a cofactor in progesterone synthesis.
- Limit caffeine and alcohol: Both can interfere with corpus luteum function.
- Regular moderate exercise: Improves insulin sensitivity, indirectly stabilising hormone levels.
A 2021 cohort of 1,200 women showed that those who adhered to a Mediterranean‑style diet had a 22% lower incidence of new fibroid development, likely due to combined anti‑inflammatory and hormone‑balancing effects.
Related Concepts and Next Steps
Understanding the progesterone‑fibroid link opens doors to exploring adjacent topics such as:
- Genetic polymorphisms affecting progesterone receptor expression.
- Impact of environmental endocrine disruptors (e.g., BPA) on luteal function.
- Role of selective estrogen receptor modulators (SERMs) in fibroid management.
- Future therapies targeting aromatase within fibroid tissue.
For readers ready to take action, start by requesting a luteal‑phase hormone panel from your GP, discuss progesterone supplementation if levels are low, and consider a pelvic ultrasound to gauge any existing fibroids.
Frequently Asked Questions
Can low progesterone cause fibroids to appear suddenly?
Progesterone deficiency doesn’t instantly create a fibroid, but it removes an important hormonal brake. Over weeks to months, unchecked estrogen can stimulate smooth‑muscle cell proliferation, leading to gradual fibroid growth that may feel sudden once symptoms surface.
How is progesterone deficiency diagnosed?
Doctors typically measure progesterone on cycle day21 (mid‑luteal). Levels below 5ng/mL are considered deficient. Some clinicians also assess the progesterone‑to‑estradiol ratio for a more nuanced picture.
Will taking progesterone supplements shrink existing fibroids?
Supplementation can slow further growth and reduce bleeding, but significant shrinkage is uncommon unless the fibroids are small and hormone‑sensitive. Combining progesterone with a SPRM or lifestyle changes often yields better results.
Is uterine artery embolization safe for women who want future pregnancies?
UAE is generally effective for symptom relief, but it carries a higher risk of reduced fertility compared with myomectomy. Women planning pregnancy usually opt for procedures that preserve the uterine lining.
What lifestyle changes have the biggest impact on progesterone levels?
Keeping a healthy body weight, reducing chronic stress, and ensuring adequate zinc and vitaminB6 intake are the most evidence‑backed actions. Regular moderate exercise and a Mediterranean‑style diet also support overall hormonal balance.

Stay consistent with progesterone support and you’ll feel better
In many cultures the balance of estrogen and progesterone is understood not merely as a biochemical phenomenon but as a reflection of the body’s natural rhythms, which have been observed for centuries in traditional practices. The modern medical literature, however, often isolates progesterone deficiency as a single variable, ignoring its interplay with lifestyle, diet, and stress. It is noteworthy that the luteal phase, when progesterone peaks, coincides with a period of relative physiological calm. When this phase is truncated, the resulting hormonal turbulence can manifest as unchecked estrogenic activity. This mechanistic insight aligns with the observations of increased fibroid incidence in women who experience chronic luteal insufficiency. Moreover, research from 2023 indicates a statistically significant correlation between low mid‑luteal progesterone and fibroid development, suggesting a causal pathway rather than mere association. The underlying molecular mechanisms involve reduced expression of anti‑proliferative genes mediated by progesterone receptors. Additionally, aromatase activity may be upregulated, fostering local estrogen synthesis within myometrial tissue. The clinical implications are profound, as therapeutic strategies that simply suppress estrogen without addressing progesterone may be incomplete. It follows that a comprehensive management plan should aim to restore progesterone levels alongside modulating estrogen. Lifestyle interventions such as weight management, stress reduction, and adequate zinc intake have been shown to support luteal function. Furthermore, micronized progesterone supplementation can re‑establish the hormonal brake, potentially slowing fibroid growth. It is also essential to consider the psychosocial dimensions, as chronic stress can perpetuate cortisol‑mediated suppression of progesterone. In summary, the relationship between low progesterone and fibroids is multifaceted, encompassing endocrine, molecular, and lifestyle factors, and warrants a holistic approach to treatment.
Oh great, another hormone “mystery” that will magically disappear if we just pop a pill 😏
Honestly, the whole “low progesterone” drama is like a soap opera in your uterus – the plot twists, the heartbreak, and the inevitable cliff‑hanger where the fibroid decides to grow just when you’re about to relax. It’s definitely not a typo that the body sometimes “forgets” to make enough progesterone, it’s just nature’s way of keepin’ us on our toes, ya know?
Progesterone acts as a brake on estrogen‑driven cell proliferation. When levels fall below the luteal‑phase threshold, the brake fails and fibroid tissue can expand. Blood tests taken on day 21 of the cycle provide a reliable measure. Supplemental micronized progesterone can restore the brake in many patients. Lifestyle factors such as obesity and chronic stress also lower progesterone production. Addressing these factors improves outcomes. Monitoring hormonal levels during treatment ensures adequacy. The goal is to achieve a balanced estrogen‑progesterone ratio.
It is with sincere concern that I acknowledge the distress many women experience when confronting hormonal imbalances. The literature underscores the importance of a methodical evaluation of luteal‑phase progesterone. A measured, evidence‑based approach, coupled with compassionate patient education, can mitigate the anxiety surrounding fibroid management.
I appreciate the thoroughness of the previous comment and would add that collaborative care-combining medical guidance with lifestyle adjustments-often yields the best results for patients seeking hormonal equilibrium.
Balancing hormones is a gradual process; stable progesterone helps keep fibroid growth in check. Simple dietary changes, like adding zinc‑rich foods, can support the corpus luteum. Regular, low‑impact exercise also contributes to hormonal health.
Let’s dissect the premise: the article claims low progesterone is the primary driver of fibroids, yet it glosses over the well‑documented role of genetic predisposition and growth factor signaling. Moreover, the language is riddled with vague qualifiers-"can" and "may"-that betray a lack of confidence in the data. If we examine the cited 2023 study, the confidence interval is wide, suggesting statistical uncertainty. A more rigorous analysis would juxtapose progesterone levels with aromatase expression, not merely present them in isolation. In short, the argument feels half‑baked, and the author should tighten both methodology and prose.
Progesterone deficiency is real.
Supporting women through hormonal challenges is a noble endeavor; when we lift each other up, the journey becomes less daunting. Remember, consistent progesterone support paired with a balanced lifestyle can turn the tide against fibroid progression. You are not alone in this fight, and every step toward hormonal harmony is a victory worth celebrating!
While the article provides a useful overview, it neglects to address the potential adverse effects of long‑term progesterone supplementation, such as increased risk of thromboembolic events. A balanced discussion should include these considerations.
The discourse surrounding hormonal interplay often descends into reductionist soundbites, overlooking the nuanced symphony of endocrine signaling. A more erudite exposition would acknowledge the heterogeneity of fibroid phenotypes and the multifactorial etiology beyond mere progesterone scarcity.
Wow!!! This whole progesterone thing is like a rollercoaster - highs, lows, and loop‑de‑loops!!! Can’t believe how much it affects everything!!! 🚀
From a mechanistic standpoint, the progesterone‑mediated downregulation of TGF‑β signaling constitutes a pivotal checkpoint in myometrial extracellular matrix remodeling, thereby attenuating fibroid expansion.
Your point about matrix remodeling is spot‑on; integrating it into treatment protocols could enhance therapeutic outcomes.
Contemplating the interplay of hormones invites a quiet reflection on how subtle biochemical shifts can sculpt our lived experience.
Data indicates that maintaining a progesterone‑to‑estradiol ratio within physiological limits correlates with reduced fibroid growth rates, underscoring the clinical relevance of routine hormonal monitoring.
It’s curious how the narrative often praises progesterone supplementation without rigorously evaluating long‑term safety; a skeptical lens reveals gaps in the evidence base.
Great summary! 😊 Looking forward to trying some of these lifestyle tweaks.